conservation and restoration of a waterlogged wooden bucket
Gert van Oortmerssen
this web publication (2007) is an adapted version of the printed version in in Paleo-Aktueel 17 (2006) (in Dutch)
The Laboratory for Conservation and Material Studies (LCM) supports the research of the Groningen Institute of Archeology (GIA) in two ways. On the one hand, important or complex finds are preserved and restored here. On the other hand, the material composition and production method of pottery and metals are examined in the context of technological research.
However, the LCM is also regularly approached by external clients. In 2004 Ernst Taayke, head of the Northern Archaeological Depot (NAD), asked to treat a waterlogged wooden bucket. Initially, the bucket would be preserved by means of a freeze drying. The costs for the most obvious conservation method were considered too high in relation to the archaeological and historical value of the object.
The bucket gave cause to investigate whether other means and methods can be used for the conservation of waterlogged archaeological wood with a limited size (up to approximately 50 x 50 x 50 cm.). After all, the GIA itself regularly finds waterlogged wood during its excavations that must be treated. Until now, this has been outsourced, with the material usually being freeze-dried.
A practical feasible conservation treatment with good results for a favorable price has been the starting piont. Two guiding principles were paramount: first, to exclude the need for after-treatment and, second, to avoid, where possible, the need for passive conservation after treatment. This contribution is a very brief discussion of alternatives to freeze drying and describes the treatment of the bucket in the course of 2005.
waterlogged wood
Conservation of waterlogged archaeological wood is considered to be one of the most challenging disciplines in the field of cultural heritage conservation. (1) Wood is only preserved in extremely dry or completely wet and low-oxygen environments. Extremely dry soils do not occur in the Netherlands, however water-saturated contexts are a normal phenomenon in the excavation practice. The uncovering of such a context greatly changes environmental conditions with exposure to oxygen, variable humidity, varying temperature and light. Especially changing the moisture content is an acute excavation risk that can lead to irreparable deformation of the wood. Secondly, there is a potential danger of mold and algae growth. This – added to the scale of some finds such as shipwrecks – illustrates the challenges of the discipline in a nutshell.
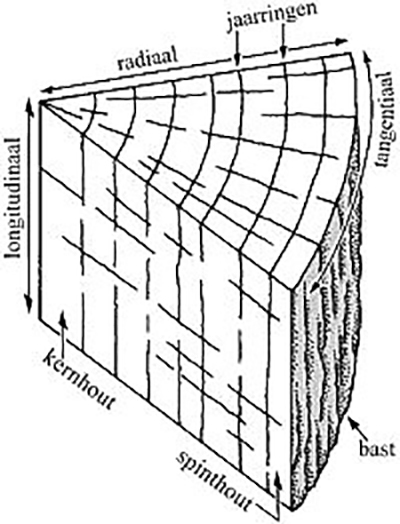
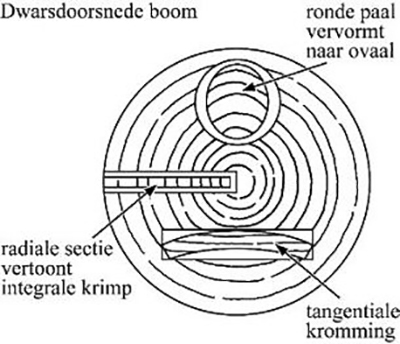
The cause of the change in shape lies in the structure of the material. Wood is anisotropic. That is: the degree of shrinkage or expansion – in response to a changing moisture content – is not the same in all directions. The wood fibers react differently in radial, tangential and longitudinal direction (fig. 1a). Even the moisture loss from a recently cut tree will lead to shape changes as shown schematically in figure 1b. Wood that has been in the soil for a long time is also exposed to chemical influences, which means that components can be leached. Weakening of the material leads to an increased risk of strong deformation upon drying out.
conservation methods
In the second half of the 20th century, various conservation methods were developed for waterlogged wood. The methods all aim to extract the water from the object, provided that the original shape is preserved. In archaeological wood, the “building blocks” of hemicellulose, cellulose and / or lignin are often so strongly affected that the shape can only be preserved by adding impregnating agents during or after removal of the water. Two techniques are most commonly used to date.
The first, and perhaps best known,technique for wood preservation is the aforementioned freeze drying (e.g. Cronyn, 1990; Oddy 1975; Schulten, 1995). The waterlogged object is cooled into a frozen state relatively quickly. Ice then sublimes from the object by artificially keeping the ambient air dry. This results in complete dewatering while retaining the shape of wood of relatively good condition. For affected wood, impregnation with a filler is necessary to prevent the material from collapsing or breaking easily. Due to the extensive equipment and the financial investment, this technique is limited and is usually applied to smaller objects. (2) An appealing example of freeze-drying on a large scale was undertaken in the port of Marseille in the early 1980s. A Roman ship has been preserved in situ there. After excavation, a building (equipped with climate control and a cooling installation) was placed around the ship. Immersion in liquid nitrogen and maintaining a low relative humidity (RH) allowed all water to be removed from the wood (Amoigen & Larrat, 1985). Due to the average high costs, research has also been carried out into freeze-drying in the air in the polar regions. The results were satisfactory, however the project was limited to wood in fair to good condition (Grattan et al, 1978 and 1980).
Impregnation with Polyethylene Glycol (PEG) is the second technique that is widely used (e.g. Pearson, 1981; Oddy, 1975). This method is based on expelling the water by means of a filler dissolved in water. The waxy substance cures in the wood during the evaporation of the water. A famous example of this application is the 17th century Swedish battleship Vasa. The entire ship was successfully preserved in a two-decade treatment. For several years, however, the condition of the wood has been threatened again by acid formation in the wood and corrosion of iron nails and bolts. The latter is a direct result of the impregnation, because PEG enhances corrosion of metals (Sändstrom et al, 2003).
In contrast to freeze drying, PEG impregnation for small objects is easily accessible in terms of means and equipment. PEG is available in a variety of molecular masses. The species with a low molecular mass and number (200-400) are liquid and can easily penetrate into the wood structure. The species with a high molecular mass and number (1500-4000) are solid in themselves, but soluble in water. The latter are less able to penetrate into the wood, but give impregnated wood the strength and stability that is desired. Experience at the LCM and descriptions from the available literature have shown that PEG treatment often results in a sticky and greasy wood surface. The “skin” of the original material is then ‘legible’ and on a greasy surface dust and dirt easily accumulates. The latter carries a risk of the need for repeated treatment. In addition, especially with hardwood, choosing the type of PEG with the correct molecular mass is not easy. The wrong choice can lead to incomplete impregnation, which means that evaporation of water left in the core can still lead to shrinkage and cracking (Cronyn, 1990; Pearson, 1981; Oddy, 1975).
Looking at the two techniques mentioned, freeze drying appears to be the most ethical; no substances need to be added that may also need to be removed. However, freezing of the wet wood leads to expansion of the water with the risk of material breakdown. PEG does not expand when freezing, which is why the wood is usually pre-washed with a low molecular mass PEG solution. Freeze-dried wood is lightweight and quite fragile, so impregnation with PEG is also used after the treatment.
Lesser known techniques are essentially variations on PEG impregnation. Some applications work on the basis of water, others on the basis of organic solvents. The “sucrose method” for example, works on the basis of refined sugar in water and is very similar to PEG impregnation (Parrent, 1985). The method was developed as a cheaper alternative to PEG and is also the cheapest in terms of material of the published treatment methods. Sugar, like the lower molecular weight PEG species, is hygroscopic when the RH rises above 65%. This makes climate control or passive conservation desirable or even necessary, so that the conservation costs can increase over time.
The “acetone / rosin method” works on the basis of an organic solvent (McKerrell et al, 1972; Oddy, 1975). The water-saturated object is immersed in it. The low surface tension of acetone results in expulsion of the water from the wood. A once solvent-saturated object can be dried if the condition of the wood is fair to good. Archaeological wood however is usually not strong enough anymore, which is why the solvent-saturated wood is immersed in a mixture of solvent and filler after dewatering. The filler cures on controlled and slow drying while retaining its shape. In this, the method is comparable to PEG impregnation.
An alternative treatment option consists of drying with an organic solvent and addition of silicone oil (Smith, 2002). Although the results are very satisfactory, the method is irreversible.
Other techniques are limited to mere dehydration; on the one hand based on solvents with a low to very low surface tension, such as the “alcohol / ether method”; on the other hand based on solvents in combination with temporary fillers as in the “alcohol / camphor method”. Until drying, the camphor provides stability in the wood, after which the camphor itself sublimates from the wood.
choice of the most appropriate conservation method
For the treatment of the bucket, the “acetone / resin method” emerges as the most interesting. Although the material costs are higher compared to sucrose or PEG impregnation, after-treatment is unnecessary. (3) In addition, treated objects can be stored or exhibited without the use of passive conservation (however where possible, passive conservation is recommended for optimal preservation).
The “acetone / rosin method” has been researched and extensively tested in the 1970s (McKerrell et al, 1972; Oddy, 1975). Waterlogged wood is immersed in acetone and, after dewatering, impregnated with a mixture of pine resin (rosin) in acetone. The results of both unaffected and degraded archaeological wood are unanimously positive. At the end of the treatment, a stable condition is created since the resin is virtually insensitive to a variable RH. In addition, the shape retention is very good and the treated wood has a natural and well “legible” appearance. Moreover, the surface is not sticky and after-treatment is unnecessary.
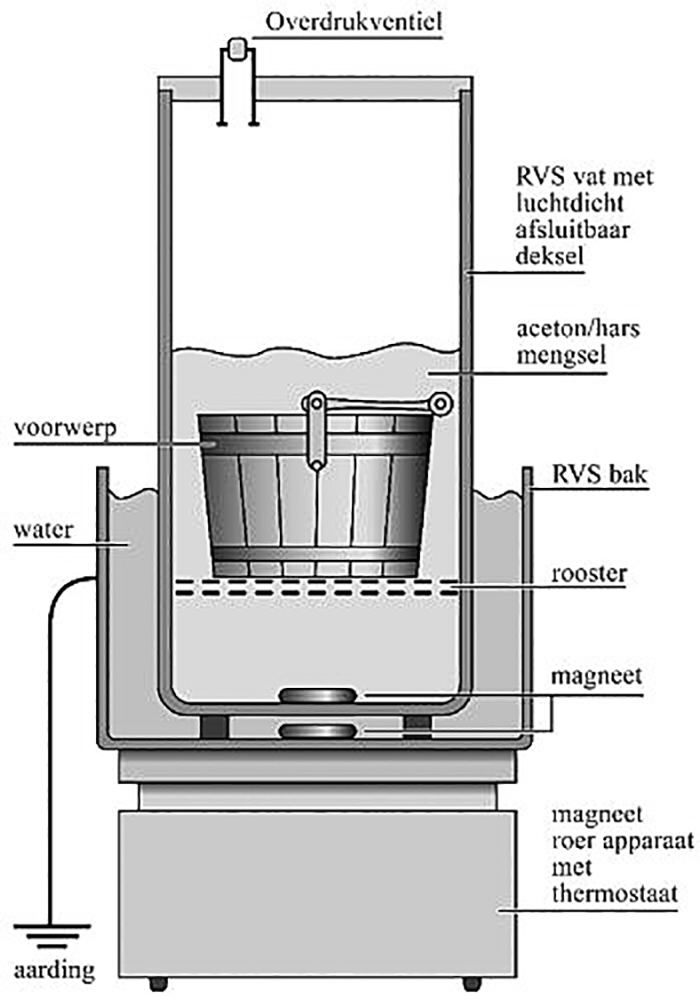
For optimal impregnation, the acetone / rosin mixture should be heated to 52o Celsius, close to the boiling point of acetone. The flammability of acetone poses an increased risk requiring safety measures. It is perhaps for this reason that the method has hardly been adopted. However, the applicability of the method focuses in this context on wood with a limited volume. In addition, the acetone / rosin mixture has a ratio that hardly builds up pressure when heated to the desired temperature. The vapor pressure of the mixture is even lower than that of pure acetone at room temperature (McKerrell et al, 1972). The application of a pressure relief valve to a vapor-tight sealed vessel in a fume hood with extraction, and measures to prevent static discharges, guarantee a safe situation during the treatment.
One of the basic ethic concepts in conservation and restoration is reversibility. The added agents (such as PEG or resin) must be removable without damaging the object. The aforementioned application of silicone oil is therefore unacceptable because of the irreversibility. In theory, PEG and rosin can be completely removed. Practice shows a different picture. Over time, some of the PEG will chemically bind to the remaining lignin and to the cell structure of the wood. Full reversibility is then no longer possible. Research on objects that were treated with the “acetone / rosin method” a few decades ago shows that rosin gradually oxidizes, which means that it may not be completely removed in the long term. The researchers recommend finding an alternative to rosin, but provisionally consider the method to be the most suitable for composite objects in which metal has been used (Esteban et al, 1998).
treatment of the bucket
The bucket (inv. No. D 2004-II.1) was accidentally found by a metal detector amateur in Spijkerboor (municipality of Aa and Hunze, Drenthe, The Netherlands) and was transferred to the Northern Archaeological Depot via provincial archaeologist W. van der Sanden. The object lay in a dilapidated but water-saturated trench of the Hunze river. It consists of twenty-one oak staves of 16 cm high, which are held together by two horizontal iron bands. (4) Apart from the loosened bottom, this object has been preserved in the original buildup. The wood can be pressed lightly and feels somewhat spongy, an indication that the condition is neither very good nor very bad. The bucket has a handle with a round recess halfway. A ring is attached through the recess with which the bucket can be hung. The suspension is fixed in the recess by means of a large knob. The handle holders on the bucket are clamped between staves and bands and attached to the staves as well by means of nails. 14C-analysis of the wood indicates a dating back to the 18th century. (5)
The iron is corroded to some extent and shows a black deposit of iron sulphides, which is characteristic of oxygen-poor soil conditions. On the iron bands and partly on the staves, however, there is local deposition of a somewhat more voluminous, brown-red corrosion. The handle is missing one of the two eyes for attachment to the bucket due to locally occurring strong corrosion. The associated eye of the handle holder on the bucket is also strongly affected.
The contractor has maintained the RH of 100% by immediately immersing the object in water in n airtight container. In this way, the bucket was delivered to the LCM. To avoid any risk of dehydration, no photos have been taken prior to treatment.
Before treatment, the storage water was measured for the presence of soluble salts, in particular chlorides. These chlorides can be harmful to the iron that is still present. However, the salt level was so low that there was no danger of chloride related iron corrosion. The bucket was brushed under running tap water with soft brushes to remove dirt and loose corrosion. It is then immersed in acetone for three weeks, with acetone being replaced every week. The bucket was then transferred to a solution of 67% rosin in acetone (w / w). (6) The resin does not completely dissolve at room temperature because the mixture is adapted to heat to 52o Celsius. The set-up was kept constant at the desired temperature in a warm water bath for four weeks (fig. 2).
After the bucket was taken out of solution, excess rosin was removed and the acetone was allowed to evaporate from the wood under controlled circumstances. Evaporation lasted about three days, but the bucket was closely monitored for two months for shape change and shrinkage at room temperature and humidity ranging from 40 to 80%. No changes have been observed and the appearance is natural (fig. 3).

The next step was to disassemble the straps, the handle and the handle holders (fig. 4). The latter have been soaked from the wood using acetone; the original nails were completely corroded. All the iron was then immersed in acetone to remove resin residues and subsequently mechanically cleaned with a grinder. In mechanical cleaning, rosin would adhere to the grinder due to frictional heat, rendering the tool unusable. The cleaned iron is then treated with tannin to create an air / moisture buffer against corrosion. Parts in contact with each other or with wood are coated with a 20% solution of Paraloid B72 in acetone / ethanol. In addition to tannin, this layer forms an additional air / moisture buffer, but it also allows wood and metal to be glued together. Parts that are visible were treated with acid free microcrystalline wax. This gives a matt to silky matt surface and it also fulfills a role as an air / moisture buffer. A covering layer of Paraloid would aesthetically detonate here due to its strong gloss. All parts have been replaced and secured with Paraloid B72. The loose bottom has also been placed back using Paraloid B72. The missing part of the handle is completed with an iron end loop (fig. 5). This end loop is attached to the original iron with a two-component adhesive and is also buffered with tannin and microcrystalline wax against corrosion. This eliminates the risk of damage to the wood by loose metal. (7)

Long-term conservation of the bucket, using passive conservation, creates a conflict of interest where the ideal values of RH are concerned. Iron should preferably be stored at an extreme but stable RH of <20%, while wood should be stored better at a slightly changing RH of 50%. The lack of chlorides and the air / moisture buffers of tannin, Paraloid B72 and microcrystalline wax present give the iron a wider bandwidth for RH. The wood has become much less hygroscopic by impregnation with rosin and can therefore also be stored within a wider RH tolerance, as long as few fluctuations occur. An RH of 35% with as little fluctuation as possible is an acceptable compromise.

conclusion
Although the “acetone / rosin method” has been satisfactorily applied to a bucket of oak in a reasonable condition, this conservation method is not readily applicable or necessary for other types of wood and stages of degradation. Some objects can suffice with dewatering by means of a solvent, while others, financial, historical or non-object related arguments can lead to the choice of an alternative method.
A disadvantage of the method used here is the relatively high cost of the resin and the use of organic solvents instead of water. The security measures also deserve more than ordinary attention. Because after-treatment is unnecessary and passive conservation is less necessary, the investment for conservation is clear and limited. Treated objects, however, must be monitored critically to understand any oxidation of the rosin and its extent to undermine the condition of the object. Where possible, rosin can be replaced by a resin that does not oxidize.
Despite the potential drawbacks outlined here, the “acetone / rosin method” is a very useful addition to the various options for preserving water-saturated wood for the LCM; especially with composite objects like wood/metal, for which PEG treatment is not an option.
postscript:
From 2010 onwards the LCM desalinates all iron objects preventively according to widely accepted guidelines.
summary: conservation of a waterlogged wooden bucket
In 2004 the Laboratory for Conservation and Material Studies (LCM) was asked to treat a waterlogged oak bucket with iron bindings. Freeze drying, the most obvious conservation technique available, was considered too costly, given the modest historical and archaeological significance of the object. The LCM took the opportunity to explore alternative methods on the basis of published research results.
Impregnation with Polyethylene Glycol (PEG) was not possible, since PEG facilitates metal corrosion. Among the alternatives the ‘acetone/rosin method’ meets the demand of stability in the face of changes in relative humidity, and it makes retreatment and passive conservation less necessary. Disadvantages are the high inflammability of the solvent and the cost of the material. Another possible drawback is the oxidation of the applied rosin (colophony), with expected increasing irreversibility over time. However, since there will be non need for retreatment, irreversibility of treatment is not considered a major problem.
The bucket has been treated according to the ‘acetone/rosin method’, with satisfactory results. The rate of resin oxidation will be monitored and, if possible, a non-oxidizing type of rosin will be applied in the future.
1 Cronyn (pp. 246-263) gives a short introduction with references. See Oddy en De Vries-Zuiderbaan as well. In addition, more recent introductions can be found via the internet using the keywords “conservation waterlogged wood”.
2 Archeoplan, Delft, The Netherlands.
3 PEG impregnation is not possible because the bucket contains iron parts. PEG eventually causes corrosion of the iron.
4 Determination of the wood species was carried out by J.N. Bottema – Mac Gillavry (GIA).
5 The dating was carried out by the Center for Isotope Research (University of Groningen) number GrA-23943: 235 ± 25 BP, calibrated (2 standard dev.) Ca. 1630 – 1949. The most likely dating is between 1733 and 1807.
6 In contrast to the usual weight / volume (w/v) recipe notation, this publication refers to a weight / weight (w/w) mixture.
7 The two-component adhesive (Araldite 2020) can be removed again using methyl chloride, the main ingredient of paint strippers. Due to the risk of migration of chlorides into the iron and the resulting risk of corrosion, the iron must be locally desalinated after removal of this adhesive.
further reading
- Amoigen, J. & P. Larrat, 1985. Traitement de bois gorgés d’eau par lyophilisation à la pression atmosphérique – Application aux objects de grande dimensions. In: Waterlogged Wood: Study and Conservation; Proceedings of the 2nd ICOM-CC Waterlogged Wood Working Group Conference. Grenoble, pp.181-188.
- Brongers, J.A. & H.F. Wijnman, 1970. The Conservation of Waterlogged Wood in the Netherlands by means of the Alcohol Ether methods. In: Berichten van de Rijksdienst voor het Oudheidkundig Bodemonderzoek. Amersfoort, pp. 321-322.
- Cronyn, J.M., 1990 (2nd edition, 1992). The elements of archaeological conservation. London, pp. 246-263.
- Esteban, L., A. Crawshaw & J.A. Spriggs, 1998. Is acetone/rosin treatment for wood reversible? In: ICOM Working Group on Wet Organic Archaeological Materials newsletter no. 29, Technical notes, pp. 3-4.
- Grattan, D.W., J.C. McCawley & C. Cook, 1978 en 1980. Potential of the Canadian Winter for freeze-drying wood, part 1 and 2; Studies in Conservation 23 (pp. 157-167) and 25 (pp. 118-136)
- Koob, S.P., 1986. The use of Paraloid B-72 as an adhesive: its application for archaeological ceramics and other materials. Studies in Conservation 31, pp. 7-14.
- McKerrell, H., E. Roger & A. Varsanyi, 1972. The acetone/rosin method for conservation of waterlogged wood. Studies in Conservation 17, pp. 111-125.
- Oddy, W.A. (ed.), 1975. Problems of the Conservation of Waterlogged Wood, (=Maritime Monographs and Reports no. 16) Londen, National Maritime Museum.
- Parrent, J.M., 1985. The conservation of waterlogged wood using sucrose. Studies in Conservation 30, pp. 63-72.
- Pearson, C., 1981. The use of polyethylene glycol for the treatment of waterlogged wood: its past, present and future. In: De Vries-Zuiderbaan (see below).
- Sandström, M., Y. Fors & I. Persson, 2003. The Vasa’s New Battle: Sulphur, Acid and Iron, (=Vasastudies no. 19), Stockholm.
- Schulten, P.J.W.M., 1995. Goed omgaan met hout; de conservering van houten voorwerpen door ‘Archeoplan’. In: K. Helfrich, J.F. Benders & W.A. Casparie (eds.), Handzaam hout uit Groninger grond. Groningen, pp. 44-47.
- Smith, C.W., 2002. A second set of experiments using hydrolyzable polymers to preserve waterlogged wood. WAG postprints, Miami, http://aic.stanford.edu/sg/wag/2002/WAG_02_cwsmith.pdf (accessed at 24 May 2006)
- De Vries-Zuiderbaan, L.H. (ed.), 1981. Conservation of Waterlogged Wood: Proceedings of the International Symposium on the Conservation of Large Objects of Waterlogged Wood, UNESCO, The Hague, The Netherlands.
2007 © GIA
