harpoons and flensing knifes: iron objects from the Smeerenburg collection – tools and products made by a smith working on an arctic whaling station
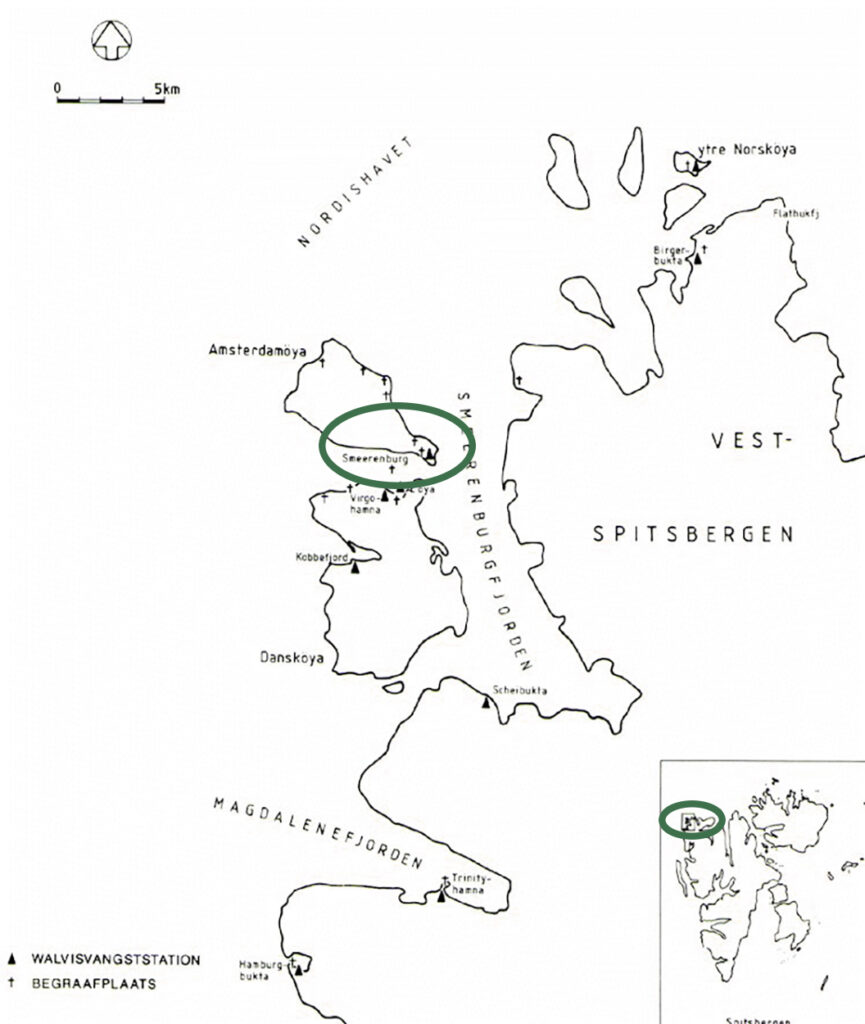
origin: Svalbard (Norway)
date: 17th century AD


During the 17th century the Republiek der Zeven Verenigde Provinciën (the Republic of Seven United Provinces) (about 1580-1795) occupied a prominent position within the network of international ecomomic relations. Whaling activities in the area of the archipelago Spitsbergen comprised an important factor. In the late 16th century the northern arctic region was explored. By among others Willem Barentsz expeditions were executed to obtain a more accessable northeastern passage. Dutch whalers have explored parts of the archipelago for decades to maintain more or less permanent storage edifices, accomodations and working areas.


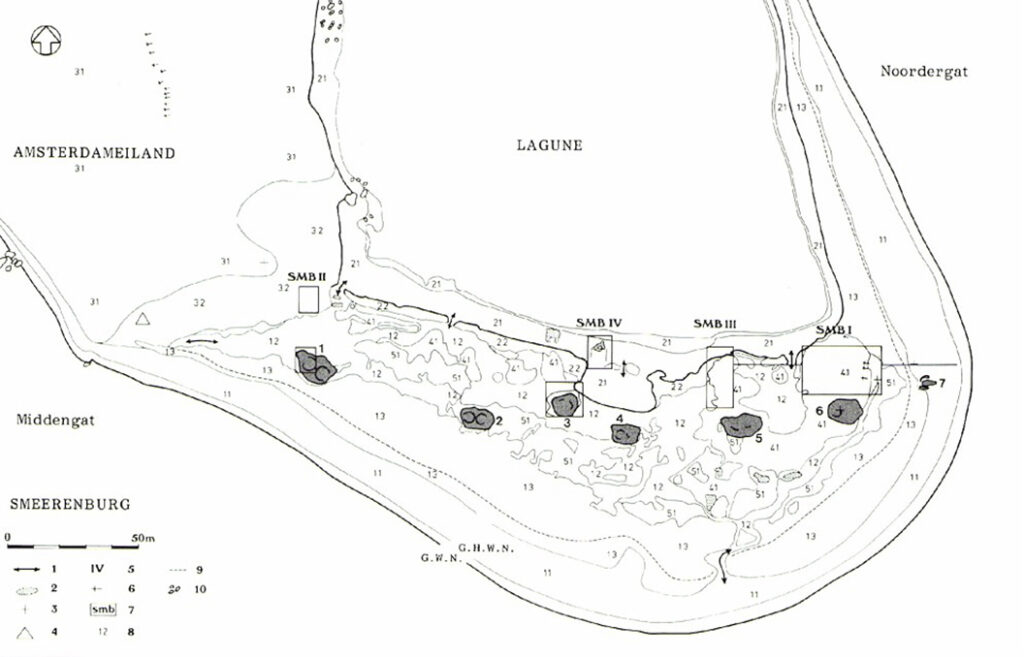
In the 1970’s and 1980’s a few intensive archaeological expeditions were executed on Amsterdameiland nearby Smeerenburg. The remains of a settlement were detected and an inventory was made. The settlement had been in use as a whaling station and was explored from 1614-1660 regularly to prepare the whales caught. To the settlement belonged houses, storage buildings and horseshoe shaped try-houses. In two of three occupation periods remains of forges were encountered. The excavated basic materials, semimanufactures, completed and repaired tools offer a detailed insight in the technical skills of a 17th century smith who had to work under spartanly circumstances. The figures show some examples of objects found on the site: before (colour) and after (b/w) treatment.

condition before treatment
The excavated iron objects from Smeerenburg have been stored for several years under disputable conditions. This stimulated corrosion. The primary corrosion products of all the Smeerenburg iron contained chlorides. Chlorides katalyse the corrosion process. Iron chloride is unstable and in presence of oxigen and water vapour corroded iron will form complexes on the border of primary corrosion and metal iron visible as brown rust. Besides rust strong yellow coloured druplets of a solution of iron ions and chlorides will form. Newly formed corrosion products will disrupt chemically stable corrosion. Fissures appear and the object starts to desintegrate. As a result of this proces the original surface and eventually the original form will disappear. As long as there is a metal core the proces will continue.
Brown coloured rust and dried in yellow droplets were present on virtually all objects. On some objects the original surface was damaged due to a loss of corrosion products.


treatment
To stop the current corrosion proces two measurements are essential: the first is a form of active conservation and consists of desalination of the iron. Desalination means removal of the chlorides. Secondly passive conservation is essential; that is to create good, dry circumstances for storage. After treatment the iron must be kept at an atmospheric humidity of less 20%.
Before treatment all objects have been photograghed in their “original” state. Moreover X-ray photo’s were made to gain information when the “original” surface was obscure.


After excavation the objects had been treated with linseed oil to reduce corrosion. This oil now hampers the desalination and must be removed first.
The iron is desalinated by means of immersion. The “bath” consisted of a solution of sodium hydroxide and sodium sulphite in destilled water. The solution promotes removal of chlorides from the iron, the iron ions form a stable complex.


After desalination the objects were allowed to dry and cleaned mechanically by means of cutting- and grinding equipment. The original surface (as far as present) is exposed. The X-ray photo’s were extremely helpful in this proces.
Fragmented objects have been reconstructed and/or completed. The restoration adhesive Paraloid B72, and epoxy adhesives as Araldite 2012 were applied. Finally the iron is impregnated with microcrystalline wax.
Objects that directly or indirectly will be exposed to air (for instance in an exhibition) have been treated with tannine, before the application of wax. Tannine will form the stable iron tannate on the iron core. This provides extra protection against corrosion.
passive conservation
After active treatment the objects are wrapped in acid free paper. Together with silicagel and atmospheric humidity indicators they are stored in air tight containers. The containers are placed in a room with climate contol.
Beside conservation, research was done on the objects to obtain information on composition and forging techniques of the iron. By means of metallographic analysis insight is gained on the technical skills of a 17th century blacksmith.
further reading
- Hacquebord, L. & W. Vroom; Walvisvaart in de gouden eeuw, opgravingen op Spitsbergen; Amsterdam, 1988 (in Dutch)
- website Arctic Center of the University of Groningen
the images on this page are originally analog and were digitized afterwards
